Atoms and Molecules
1) What Are Atoms? Why Are They Called the Basic Structures of Matter?
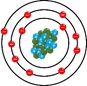
- Atoms are the smallest indivisible parts of matter.
- Atoms are elementary particles which come together to form molecules which combine to form matter.
- Since an atom cannot be divided further into small particles, it is called the basic structure of matter.
2) Does the Atom or the Molecule of a Particular Matter Have Its Own Colour?
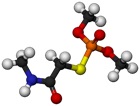
- Atom or molecule does not reflect a visible light that the eyes can see.
- We cannot see the colour of a single atom or molecule, because it is too faint to be noticed by the eyes.
- Hence it can be concluded that they do not have a specific colour.
3) Why Do Oil and Water Not Mix?

- For any liquid to dissolve in water, it should be capable of breaking the bonds between the atoms of a water molecule.
- This cannot be done by the fat or oil molecules found in oil; hence it does not dissolve in water.
4) Aluminium and Iron Are Both Metals. 1 kg of Aluminium Needs More Space Than 1 kg of Iron. Why So?
- Iron occupies less space than aluminium as the molecules of iron metal are compactly packed, making iron dense.
- In the case of aluminium, the molecules are loosely packed. Hence, they occupy more space as compared to iron molecules.
Share

