Maximum number of electron in K-shell = 2
Maximum number of electron in L-shell = 8
If K and L-shells of an atom are full,
then the total number of electrons in the atom would be (2 + 8) = 1 electrons.
Chapter 4 of NCERT Class 9 Science discusses the structure of atoms, focusing on subatomic particles: protons (positive), neutrons (neutral), and electrons (negative). It explains how protons and neutrons form the dense nucleus, while electrons occupy energy levels around it. The atomic number defines an element, while the mass number is the total of protons and neutrons.
The NCERT Class 9 Science Chapter 4 – Structure of Atoms are tailored to help the students master the concepts that are key to success in their classrooms. The solutions given in the PDF are developed by experts and correlate with the CBSE syllabus of 2025-2026. These solutions provide thorough explanations with a step-by-step approach to solving problems. Students can easily get a hold of the subject and learn the basics with a deeper understanding. Additionally, they can practice better, be confident, and perform well in their examinations with the support of this PDF.
Download PDF
Students can access the NCERT Class 9 Science Chapter 4 – Structure of Atoms. Curated by experts according to the CBSE syllabus for 2025–2026, these step-by-step solutions make Science much easier to understand and learn for the students. These solutions can be used in practice by students to attain skills in solving problems, reinforce important learning objectives, and be well-prepared for tests.
If K and L shells of an atom are full, then what would be the total number of electrons in the atom?
Maximum number of electron in K-shell = 2
Maximum number of electron in L-shell = 8
If K and L-shells of an atom are full,
then the total number of electrons in the atom would be (2 + 8) = 1 electrons.
How will you find the valency of chlorine, sulphur and magnesium?
If the number of electrons in the outermost shell is less than 4 then,
Valency of an atom = number of electrons in the outermost shell of the atom.
i. In the case of magnesium,
Thus, the valency of magnesium = 2
If the number of electrons in the outermost shell is less than 4 then,
Valency of an atom = 8 – Number of electrons in the outermost shell.
ii. In case of sulphur,
The valency of sulphur = 8 − 6 = 2
iii. In case of chlorine,
The valency of chlorine = 8 −7 = 1
With the help of Table 4.1, find out the mass number of oxygen and sulphur atom.
Mass number = Number of protons + Number of neutrons
Mass number of O2=8+8=16
Mass number of S=16+16=32
Write the electronic configuration of any one pair of isotopes and isobars.
Two isotopes of carbon are :
612C
614C
The electronic configuration of C612C is 2,4
The electronic configuration of C614C is 2,4
Two isobars of carbon are :
2040Ca
1840Ar
The electronic configuration of 2040Ca is 2, 8, 8, 2.
The electronic configuration of 1840Ar is 2, 8, 8.
If the number of electrons in an atom is 8 and the number of protons are also 8, then
(i) What is the atomic number of the atom and (ii) What is the charge on the atom?
(i) The atomic number = number of protons.
Hence, the atomic number of the atom is 8.
(ii) Since the number of both electrons and protons is equal, therefore, the charge on the atom is 0 i.e., it is a neutral atom.
Helium atom has an atomic mass of 4 u and two protons in its nucleus. How many neutrons does it have?
Number of protons in Helium Atom =2
Atomic Mass = Number of Protons + Number of Neutrons
4=2+ Number of Neutrons
4=2+ Number of Neutrons
Number of Neutrons = 4 - 2 = 2
Number of Neutrons = 4 - 2 = 2
Draw a sketch of Bohr’s model of an atom with three shells.
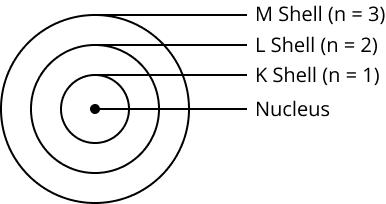
Name the three subatomic particles of an atom.
Three subatomic particles of an atom are as follows:
i. Proton
ii. Electron
iii. Neutron.
What are canal rays?
Canal rays are beams of positively charged ions. It was discovered in 1886 by Goldstein. Canal rays are also known as anode rays.
If an atom contains one electron and one proton, will it carry any charge or not?
An atom carrying one electron and one proton will carry no charge as the negatively charged particle will combine with the positively charged particles and the overall magnitude of the atom will be zero.
On the basis of Thomson’s model of an atom, explain how the atom is neutral as a whole.
According to Thomson’s model of the atom:
i. An atom consists of both negatively and positively charged particles.
ii. The negatively charged particles are embedded in the positively charged sphere.
iii. These negative and positive charges are equal in magnitude.
iv. They counterbalance each other’s effect and make an atom neutral.
On the basis of Rutherford’s model of an atom, which subatomic particle is present in the nucleus of an atom?
According to Rutherford’s model of an atom, protons are present in the nucleus of an atom.
For the symbols H, D and T tabulate three sub-atomic particles found in each of them.
|
Symbol |
Electron |
Proton |
Neutron |
|
H |
1 |
1 |
0 |
|
D |
1 |
1 |
1 |
|
T |
1 |
1 |
2 |
Write the distribution of electrons in carbon and sodium atoms?
Atomic number of carbon = 6= Number of protons = Number of electrons
The distribution of electrons in carbon atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 4 electrons
or, we can write the distribution of electrons in a carbon atom as 2,4
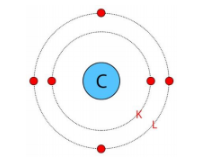
Atomic number of sodium = 11= Number of protons = Number of electrons
The distribution of electrons in sodium atom is given by:
First orbit or K-shell = 2 electrons
Second orbit or L-shell = 8 electrons
Third orbit or M-shell = 1 electron
Or, we can write the distribution of electrons in a sodium atom as 2, 8,1
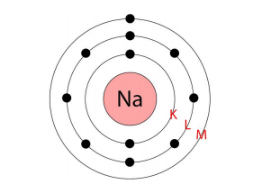
What are the limitations of J.J. Thomson’s model of the atom?
Limitations of J.J. Thomson’s model of the atom.
It fails to explain the stability of an atom.
It doesn’t talk about the nucleus of an atom.
It failed to explain the reason for positive and negative charges binding together.
It also doesn’t explain Rutherford’s model.
What are the limitations of Rutherford’s model of the atom?
Rutherford’s model of the atom fails to explain the stability of an atom. He argued that electrons move in a circular path called the orbit. The Revolution of electrons in the orbit will radiate energy which will make the atom unstable and electrons will fall inside the nucleus. But in reality this is not the case and Rutherford’s model fails to explain the reason for the same.
Na+ has completely filled K and L shells. Explain.
Atomic number of Na 11 Total number of electrons The electronic configuration of Na = 2, 8, 1 The electronic configuration of Nation 2 (K-shell), 8 (L-shell) Thereby Na+ ion has completely filling K and L shells.
if bromine atom is available in the form of, say, two isotopes Br(49.7%) and Br(50.3%) calculate the average atomic mass of bromine atom.
Average atomic mass of Bromine atom
79x49.7+ 81 x 50.3 100
3926.3+4074.3
100
8000.6
100
80.006
≈80u
If Z = 3, what would be the valency of the element? Also, name the element.
Atomic number = Z = 3.
Its electronic configuration is 2, 1.
Hence, the valency of the element is 1
(Since the outermost shell has only one electron).
Therefore, the element with Z = 3 is lithium (Li).
For the following statements, write T for ‘True’ and F for ‘False’.
a). J.J. Thomson proposed that the nucleus of an atom contains only nucleons.
Ans: False
b). A neutron is formed by an electron and a proton combining together. Therefore, it is neutral.
Ans: False
c). The mass of an electron is about 1/2000 times that of the proton.
Ans: True
d). An isotope of iodine is used for making tincture iodine, which is used as a medicine.
Ans: False
Isotopes of an element have
The same physical properties
Different chemical properties
Different number of neutrons
Different atomic numbers
c) Different numbers of neutrons.
Isotopes are atoms of the same element having the same atomic number, but different mass numbers.
Number of valence electrons in Cl−1 ion is:
16
8
17
18
Atomic number of Cl = 17
Electronic configuration of Cl = 2, 8, 7
Electronic configuration of Cl−1 ion = 2, 8, 8
Thus, the number of valence electrons in Cl−1 ion = 8
Which one of the following is a correct electronic configuration of sodium?
2, 8
8, 2, 1
2, 1, 8
2, 8, 1
Atomic number of sodium = = Number of electrons
So, electronic configuration of sodium = 2, 8, 1.
Complete the following table.
|
Atomic Number |
Mass Number |
Number of Neutrons |
Number of Proton |
Number of Electrons |
Name of the atomic species |
|
9 |
- |
10 |
- |
- |
- |
|
16 |
32 |
- |
- |
- |
Sulphur |
|
- |
24 |
- |
12 |
- |
- |
|
- |
2 |
- |
1 |
- |
- |
|
- |
1 |
0 |
1 |
1 |
- |
|
Atomic Number |
Mass Number |
Number of Neutrons |
Number of Proton |
Number of Electrons |
Name of the atomic species |
|
9 |
19 |
10 |
9 |
9 |
Fluorine |
|
16 |
32 |
16 |
16 |
16 |
Sulphur |
|
12 |
24 |
12 |
12 |
12 |
Magnesium |
|
1 |
2 |
1 |
1 |
1 |
Deuterium |
|
1 |
1 |
0 |
1 |
1 |
Protonium |
Compare the properties of electrons, protons and neutrons.
The difference between electron, proton and neutrons are as follows:
|
Electron |
Proton |
Neutron |
|
1. It is present outside of the nucleus of an atom. |
1. It is present inside the nucleus of an atom. |
1. It is present inside the nucleus of an atom. |
|
2. Carry negative charge. |
2. Carry positive charge |
2. It is neutral. |
|
3. Its weight is negligible. |
3. It weighs around 2000 times as mass of electrons. |
3. Weight is the same as a proton. |
Compare all the proposed models of an atom given in this chapter.
Comparison of all models of an atom are given below:
|
Thomson’s Model |
Rutherford’s Model |
Bohr’s Model |
|
1. According to this mode positively and negatively charged ions are embedded throughout the atom. |
1. This model explained that all the positive ions are embedded in the nucleus and negative ions revolve around it. |
1. Electron or negatively charged particles move around in a fixed circular path called the orbit. There is no energy generation in the electron revolution. |
Define valency by taking examples of silicon and oxygen.
Valency of an element is defined as the number of electrons in its outermost shell.
In the case of silicon,
Outermost shell electron = 4
If the number of electrons in the outermost shell of the atom of an element is less than or equal to
4,
Valency of an atom = number of electrons in the outermost shell
Thus, the valency of silicon = 4.
In the case of oxygen,
Outermost shell electron = 6
If the number of electrons in the outermost shell of the atom of an element is greater than 4,
Then, the valency of an atom = 8 – Number of electrons in the outermost shell.
Thus, the valency of oxygen is (8 − 6) = 2
Explain with examples.
i. Atomic number
ii. Mass number,
iii. Isotopes
iv. Isobars.
Give any two uses of isotopes.
i. Atomic Number: Total number of protons present in the atom of an element is called the atomic number of that element.
Example: Oxygen has 8 protons. Thus, the atomic number of Oxygen is 8.
ii. Mass Number: The sum of the number of protons and neutrons present in the atom of an element is called the mass number.
Example: Oxygen has 8 protons and 8 neutrons.
Therefore the mass number of oxygen is 8+8=16
iii. Isotopes: Isotopes are atoms of the same element having the same atomic number, but different mass numbers.
Example Three isotopes of Hydrogen are
1) Protium (H)
2) Deuterium (H)
3) Tritium (H)
(iv). Isobars: Isobars are atoms with the same mass number but different atomic numbers, le, isobars are atoms with the same mass number from different elements
Example: 40/20 Ca and 40/18 Ar are two isobars.
Composition of the nuclei of two atomic species X and Y are given as under X Y
|
X |
Y |
|
|
Protons |
6 |
6 |
|
Neutrons |
6 |
8 |
Give the mass numbers of X and Y. What is the relation between the two species?
Mass number = Number of protons + Number of neutrons
Mass number of X = 6 + 6 = 12
Mass number = Number of protons + Number of neutrons
Mass number of Y = 6 + 8 = 14
Atomic number = Number of protons
The atomic number of X= 6 = Atomic number of Y
These two atomic species X and Y have the same atomic number, but different mass numbers. Hence, they are isotopes.
Describe Bohr’s model of the atom.
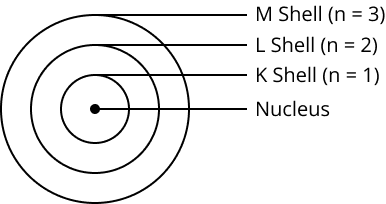
Describe Bohr’s model of the atom.

Summarize the rules for the writing of the distribution of electrons in various shells for the first eighteen elements.

Admissions Open for
Admissions Open for
The NCERT solution for Class 9 Chapter 4: Structure of Atoms is important as it provides a structured approach to learning, ensuring that students develop a strong understanding of foundational concepts early in their academic journey. By mastering these basics, students can build confidence and readiness for tackling more difficult concepts in their further education.
Yes, the NCERT solution for Class 9 Chapter 4: Structure of Atoms is quite useful for students in preparing for their exams. The solutions are simple, clear, and concise allowing students to understand them better. They can solve the practice questions and exercises that allow them to get exam-ready in no time.
You can get all the NCERT solutions for Class 9 Science Chapter 4 from the official website of the Orchids International School. These solutions are tailored by subject matter experts and are very easy to understand.
Yes, students must practice all the questions provided in the NCERT solution for Class 9 Science Chapter 4: Structure of Atoms as it will help them gain a comprehensive understanding of the concept, identify their weak areas, and strengthen their preparation.
Students can utilize the NCERT solution for Class 9 Science Chapter 4 effectively by practicing the solutions regularly. Solve the exercises and practice questions given in the solution.
CBSE Schools In Popular Cities