NCERT Solutions for Class 11 Chemistry Part 2 chapter 1 – Redox Reaction
As students are growing up, they deserve something that would enable them to learn everything. One such important chapter which is included in this solution provider guide is class 11 chemistry part 2 chapter 1. Further, details on Redox Reactions are explained at length in this important chapter on oxidation and reduction reactions. From seeking a clear explanation to just revising the content, the solution with class 11 chemistry part 2 chapter 1 PDF has a comprehensive answer to all your queries.
Access Answers to NCERT Solutions for Class 11 Chemistry Part 2 chapter 1 – Redox Reaction
Students can access the NCERT Solutions for Class 11 Chemistry Part 2 chapter 1 – Redox Reaction. Curated by experts according to the CBSE syllabus for 2023–2024, these step-by-step solutions make Chemistry much easier to understand and learn for the students. These solutions can be used in practice by students to attain skills in solving problems, reinforce important learning objectives, and be well-prepared for tests.
Redox Reaction
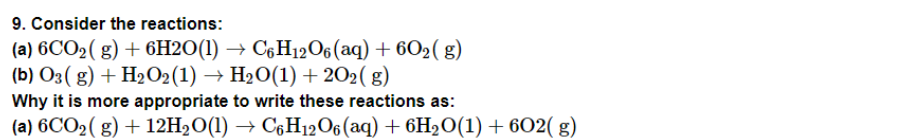
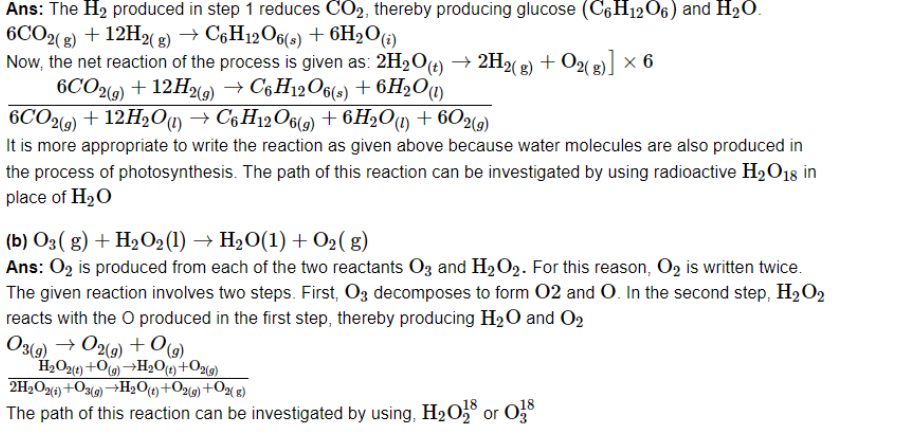


Assign oxidation numbers to the underlined elements in each of the following species:
(a) NaH2PO4
Ans: P's oxidation number will be x.
We are aware of this.
Oxidation number of Na=+1
Oxidation number of H=+1
Oxidation number of O=−2
(+1)(+1)(x)(−2)=NaH2PO4
Then there's
1(+1)+2(+1)+1(x)+4(−2)=0
1+2+x−8=0
x=+5
As a result, P's oxidation number is +5
(b) NaHSO4
(+1)(+1)(x)(−2)
Ans:
NaHSO4
Then there's
1(+1)+1(+1)+1(x)+4(−2)=0
1+1+x−8=0
x=+6
As a result, S's oxidation number is +6
(c) H4P2O7
Ans:
H4P2O7
Then, there's
4(+1)+2(x)+7(−2)=0
4+2x−14=0
2x=+10
x=+5
As a result, P's oxidation number is +5
(d) K2MnO4
Ans: Then, there's
2(+1)+x+4(−2)=0
2+x−8=0
x=+6
As a result, Mn 's oxidation number is +6
(e) CaO2
Ans:
Then, there's
(+2)+2(x)=0
2+2x=0
x=-1
As a result, O's oxidation number is −1
(f) NaBH4
Ans: Then, there's
1(+1)+1(x)+4(−1)=0
1+x−4=0
x=+3
As a result, B's oxidation number is +3
(g) H2 S2O7
Ans:
H2 S2O7
Then, there's
2(+1)+2(x)+7(−2)=0
2+2x−14=0
2x=12
x=+6
As a result, S's oxidation number is +6
(h) Kl(SO4)2.12H2O
Ans:
KAl(SO4)2.12H2O
Then, there's
1(+1)+1(+3)+2(x)+8(−2)+24(+1)+12(−2)=0
1+3+2x−16+24−24=0
2x=12
x=+6
As a result, S's oxidation number is +6
Because water is a neutral molecule, we can disregard it. The sum of all atoms in the water molecule's oxidation numbers can then be considered as zero. As a result of disregarding the water molecule, we now have
Balance the following equations in basic medium by ion-electron method and oxidation number methods and identify the oxidizing agent and the reducing agent.
P4(s)+OH(aq)−→PH3g+HPO2(aq)−
(a) The oxidation number of P drops from 0 to −3 in P4 and increases from 0 to +2 in HPO2. As a result, P4 serves as both an oxidizing and reducing agent in this process. Ion-electron method:
The half-equation for oxidation is:
P4( s)→HPO2(aq)−
P atom is balanced in the following way:
P4( s)0→4HPO2(aq)−
The O.N. is balanced by adding eight electrons in the following way:
P4( s)→4HPO2(a)+8e−
The charge is balanced by the addition of 12OH− as follows:
P4( s)+12OH(aq)−→4HPO2(aq)−+8e−
By adding 4H2O, the H and O atoms are balanced.
P4(s)+12OH(aq)−→4HPO2(aq)−+4H2O(1)+8e−…..(i)
The half-reduction equation is as follows:
P4( s)+PH3( g)
The P atom is in a state of equilibrium.
By adding 12 electrons to the Q.N., it is balanced:
P4( s)+12e−→4PH3( g)
The charge is balanced by the addition of 12 OH−as follows:
P4( s)+12e−→4PH3( g)+12OH(aq)−
12H2O is used to balance the 0 and H atoms as follows:
P4(s)+12H3O(1)+12e−→4PH3( g)+12OH(2q)− (ii)
The balanced chemical equation can be found by multiplying equations ∣ and (ii) by 3 and then adding them.
5P4( s)+12H2O(i)+12HO(aq)−→8PH3( g)+12HPO(aq)−
(b) N2H4(l)+ClO3−(aq)→NO(g)+Cl−(g)
Ans:
N′ s oxidation number rises from−2 in N2H4 to +2 in NO, while Cl 's oxidation number falls from +5 in ClO3 to Cl−.As a result, N2H4 is the reducing agent and ClO3 is the oxidizing agent in this reaction.
Ion-electron method:
The half-equation for oxidation is:
N2H4(1)→NO(g)
The N atoms are balanced in the following way:
N2H4(1)→2NO(g)
By adding 8 electrons to the oxidation number, the oxidation number is balanced:
N2H4(l)→2NOfg)+8e−
8OH−ions are added to balance the charge as follows:
N2H4(I)+8OH(aq)−→2NO(g)+8e−
6H2O is added to balance the O atoms as follows:
N2H4(1)+8OH(aq)−→2NO(g)+6H2O+8e−….
The half-reduction equation is as follows:
ClO−+53(aq)−1→Cl(aq)−
By adding 6 electrons to the oxidation number, the oxidation number is balanced:
ClO3(aq)+6e−→Cl(aq)−
6OH−ions are added to balance the charge as follows:
ClO3(aq)−+6e−→Cl(aq)−+6OH(aq)−
By adding 3H2O as follows, the O atoms are balanced.
Equation I is multiplied by 3 and equation (ii) is multiplied by 4, resulting in the balanced equation:
3 N2H4(1)→6NO(g)+4Cl(aq)−+6H2O(l)
Oxidation number method:
Total reduction in N oxidation number N=2×4=8
Total reduction in Cl oxidation number Cl=1×6=6
To balance the rise and decrease in O.N., multiply
N2H4 by three and ClO3 by four.
3 N2H4(l)→4ClO3(aq)−→NO(g)+Cl(aq)−
The atoms of N and Cl are balanced as follows:
3 N2H4(1)→4ClO3(aq)−→6NO(g)+4Cl(aq)∘
6H2O is added to balance the O atoms as follows:
3 N2H4(1)→4ClO3(aq)→6NO(g)+4Cl(aq)−+6H2O
This is the equation that must be balanced.
(c) Cl2O7( g)+H2O2(αq)⟶ClO2(αq)−+O2(g)+H(c)+
Ans: The oxidation number of Cl decreases from +7 in Cl2O7 to +3 in ClO2 and the oxidation number of O increases from H2O2 to zero in O2. Hence, in this reaction, Cl2O7 is the oxidizing agent and H2O2 is the reducing agent. Ion-electron method:
The half-equation for oxidation is:
H2O2(aq)→O2( g)0
By adding two electrons to the oxidation number, the oxidation number is balanced as follows:
H2O2(aq)→O2( g)+2e−
2OH - ions are added to balance the charge as follows:
H2O2(aq)+2OH2( g)−→O2( g)+2e−
By adding 2H2O2 as follows, the oxygen atoms are balanced.
H2O2(aq)+2OH2( g)−→O2( g)+2H2O2(t)+2e−….(i)
The half-reduction equation is as follows:
The Cl atoms are balanced in the following way:
Cl2O7( g)→ClO2(aq)−
By adding 8 electrons to the oxidation number, the oxidation number is balanced:
Cl2O7( g)+8e−→2ClO2(aq)−
6OH' is added to balance the charge as follows:
Cl2O7( g)+8e−→2ClO2(aq)−+6OH−(aq)
By adding 3H2O as follows, the oxygen atoms are balanced.
Cl2O7( g)+3H2O+8e−→2ClO2(aq)−+6OH(aq)−…. (ii)
By multiplying equation (i) by 4 and adding equation (ii) toit, you can get the balanced equation.
Cl2O7( g)+4H2O2(aq)+2OH(aq)−→2ClO2(aq)−+4O2( g)+5H2O(c)
Method for calculating the oxidation number:
The total number of oxidations has decreased
Cl2O7=4×2=8
The total number of oxidations has decreased
H2O2=2×1=2
To balance the rise and decrease in the oxidation number, multiply H2O2 and O2 by 4.
Cl2O7Vg+4H2O2(aq)→ClO2(aq)−+4O2( g)
The Cl atoms are balanced in the following way:
Cl2O7( g)+4H2O2(aq)→2ClO2(aq)−+4O2( g)
The O atoms are balanced by adding 3H2O in the following way:
Cl2O7( g)+4H2O2( aq )→2ClO2(aq)−+4O2 ge+3H2O41 2OH−and 2H2O are used to balance the H atoms as follows:
Cl2O7( g)+4H2O2(aq)+2OH−→2ClO2(aq)−+4O2( g)+5H2O(l)
This is the equation that must be balanced.
What are the oxidation numbers of the underlined elements in each of the following and how do you rationalize your results?
(a) KI3
Ans: In KI3 K has an oxidation number (O.N.) of one. As a result, I's average oxidation number is 13.0.N, on the other hand, cannot be fractional. To determine the oxidation states, we must first study the structure of KI3.
An iodine atom makes a coordinate covalent link with an iodine molecule in a KI3 molecule.
K++1[I0−I0←I−1]
As a result, the O.N. of the two atoms that make up the I2 molecule in a KI3 molecule is 0, whereas the 0.N. of the I atom that makes up the coordinate bond is −1.
(b) H2 S4O6
Ans:
Now, 2(+1)+4(x)+6(+2)=0
⇒2+4x−12=0
⇒4x=10
⇒x=+2
⇒x=+212
However, O.N. cannot be fractional. Hence, S must be present in different oxidation states in the molecule.
The O.N. of two of the four S atoms is +5 and the O.N. of the other two S atoms is 0.
(c) Fe3O4
Ans: When the O.N. of O is set to −2, the O.N. of Fe is found to be +223. O.N., on the other hand, cannot be fractional. One of the three Fe atoms in this example has an O.N. of +2, whereas the other two Fe atoms have an O.N. of FeO+2Fe2O3
(d) CH3CH2OH
Ans:
x+1−2
C2H6O
2(x)+6(+1)+1(−2)=0
2x+6−2=0
x=−2
This molecule's two carbon atoms are found in two separate settings. As a result, their oxidation numbers cannot be the same.
As a result, C has the oxidation states of -3 and -1.
(e) CH3COOH
Ans:
x+1−2
C2H4O2
2(x)+4(+1)+2(−2)=0
2x+4−4=0
x=0
The average O.N. of
C, on the other hand, is 0 . This molecule's two carbon atoms are found in two separate settings. As a result, their oxidation numbers cannot be the same.
In CH3COOH,C has the oxidation states of +3 and −3.
Justify that the following reactions are redox reactions:
(a) CuO(s)+H2( g)→Cu(s)+H2O(g)
Ans: Let's write the oxidation number of each element in the reaction as follows:
CuO(s)+H2( g)→Cu(s)+1−2
Cu 's oxidation number falls from +2 in CuO to 0 in Cu, implying that CuO is reduced to Cu.
In addition, the oxidation number of H2 increases from 0 to +1 in H2O, indicating that H2 is oxidized to H2O. As a result, this is a redox reaction.
(b) Fe2O3( s)+3CO(g)→2Fe(s)+3CO2( g)
Ans: Let's write the oxidation number of each element in the reaction as follows:
Fe2O3( s)+3CO(g)→2Fe(s)+3CO2( g)
Fe 's oxidation number falls from +3 in Fe2O3 to 0 in Fe, implying that Fe2O3 is reduced to Fe. The oxidation number of C, on the other hand, increases from +2 in CO to +4 inCO2, indicating that CO is oxidized to CO2. As a result, the reaction in question is a redox reaction.
(c)
4BCl3( g)+3LiAlH4( s)→2 B2H6( g)+3LiCl(s)+3AlCl3( s)
Ans: Let's write the oxidation number of each element in the reaction as follows:
4BCl3( g)+3LiAlH4( s)→2 B2H6( g)+3LiCl(s)+3AlCl3( s)
The oxidation number of B drops from +3 in BCl3 to −3 in B2H6 in this reaction.
BCl3 is reduced to B2H6 in this way. In addition, the oxidation number of H in LiAlH4 increases
−1 in B2H6, indicating that LiAlH 4 is oxidized to B2H6. As a result, the reaction in question is a redox reaction.
(d) 2 K( s)+F2( g)→2 K+F−(s)
Ans: Let's write the oxidation number of each element in the reaction as follows:
2 K( s)+F2( g)→2 K+F−(s)
The oxidation number of K increases from 0 in to +1 in KF in this reaction, indicating that K is oxidized to KF. The oxidation number of F, on the other hand, decreases from 0 in F2 to −1 in
KF, indicating that F2 is reduced to KF.
As a result, the preceding reaction is a redox reaction.
(e) 4NH3( g)+5O2( g)→4NO(g)+6H2O(g)
Ans:
Let's write the oxidation number of each element in the reaction as follows:
−3+10
4NH3( g)+5O2( g)→4
NO(g)+6H2O(g)
The oxidation number of N rises from −3 in NH3 to +2 in NO in this case. The oxidation number of O2 drops from 0 in −2 NO and H2O, indicating that O2 is reduced. As a result, the reaction in question is a redox reaction.
Fluorine reacts with ice and results in the change:
H2O(s)+F2( g)→HF(g)+HOF(g)
Justify that this reaction is a redox reaction.
Let's write the oxidation number of each atom in the reaction above its symbol as follows:
+1−20+1⋅1+1⋅2+1
H2O(s)+F2( g)→ HF(g)+HOF(g)
Here, we have observed that the oxidation number of F increases from 0 in F2 to +1 in HQF . Also, the oxidation number decreases from 0 in F2 to−1 in HF. Thus, in the above reaction,
F is both oxidized and reduced. Hence, the given reaction is a redox reaction.
Calculate the oxidation number of Sulphur, chromium, and nitrogen in H2SO5, Cr2O72− and NO3−. Suggest structure of these compounds. Count for the fallacy.
(i) H2SO5
2(+1)+1(x)+5(−2)=0
2+x−10=0
x=+8
S's O.N., on the other hand, cannot be +8. S has six electrons in its valence shell. As a result, S's O.N. cannot be greater than +6.
The structure of H2SO5 is depicted in the diagram below.
2(H)+1( S)+β(0)+2(0 in peroxy linkage)
2(+1)+1(x)+3(−2)+2(−1)=0
2+x−6−2=0
x=+6
As a result, S's O.N. is +6.
(ii) x−2 Cr2O72−
2(x)+7(−2)=−2
2x−14=−2
x=+6
The O.N. of Cr in Cr2O72− is not a fallacy in this case.
The structure of Cr2O72− is depicted in the diagram below
Each of the two Cr atoms here have an O.N. of +6
(iii) NO3
1(x)+3(−2)=−1
x−6=−1
x=+5
The O.N. of N inNO3∘ is not a fallacy in this case. The structure of NO3∘ is depicted in the diagram below
The O.N. value of the N atom is +5.
Write the formula for the following compounds:
(a) Mercury (II) chloride
Ans:
HgCl2
(b) Nickel (II) sulphate
Ans:
NiSO4
(c) Tin (IV) oxide
Ans:
SnO2
(d) Thallium(I) sulphate
Ans:
Tl2SO4
(e) Iron (III) sulphate
Ans:
Fe2(SO4)3
(f) Chromium (III) oxide
Ans:
Cr2O3
Suggest a list of the substances where carbon can exhibit oxidation states from –4 to +4 and nitrogen from –3 to +5.
|
Substance |
O.N. of carbon |
|
CH2Cl2 |
0 |
|
ClC≡CCl |
+1 |
|
HC=CH |
–1 |
|
CHCl3,CO |
+2 |
|
CHCl3 |
–2 |
|
Cl3C−CCl3 |
+3 |
|
H3C−CH3 |
–3 |
|
CCl4,CO2 |
+4 |
|
CH4 |
–4 |
The substances where nitrogen can exhibit oxidation states from –3 to +5 are listed in the following table.
|
Substance |
O.N. of carbon |
|
N2 |
0 |
|
N2O |
+1 |
|
N2H2 |
–1 |
|
NO |
+2 |
|
N2H4 |
–2 |
|
N2O3 |
+3 |
|
NH3 |
–3 |
|
NO2 |
+4 |
|
N2O5 |
+5 |
The oxidation number (O.N.) of S in sulphur dioxide (SO2) is +4, while the O.N. of S can range from +6 to −2.
As a result, SO2 can function as both an oxidizing and a reducing agent. The O.N. of O in hydrogen peroxide (H2O2) is −1, and the range of O.N. that O can have is 0 to −2. The oxidation values +1 andm+2 are also possible form O.
As a result,
H2O2 can function as both an oxidizing and a reducing agent. As a result, in this scenario, the O.N. of O can only drop. As a result, O3 serves solely as an oxidant.
The O.N. of (HNO3) is +5, and the range of O.N that N can have from +5 to−3. As a result, in this scenario, the O.N. of N can only drop. As a result, HNO, serves solely as an oxidant.
Whenever a reaction between an oxidizing agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidizing agent is in excess. Justify this statement giving three illustrations.
When an oxidizing agent and a reducing agent react, a lower oxidation state compound is formed if the reducing agent is in excess, and a higher oxidation state compound is formed if the oxidizing agent is in excess. As an example, consider the following:
(i) Reducing and oxidising agents, respectively, are P4 and F2. When an excess of P4 is treated with F2,PF3 is formed, with a positive oxidation number (O.N.) for P. However, if P4 is treated with an excessive amount of F2,PF5 is formed, with a P.N. of +5.
(ii) O2 is an oxidising agent, whereas K is a reducing agent. K2O is generated when an excess of
K reacts with O2, with the O.N. of O being −2.
4 K( excess )+O2→2 K2O
When K reacts with an excess of O2, however, 2 K2O2 is produced, with the O.N. of O being
4 K+O2( excess )→2 K2O2
While C is a reducing agent, O2 is an oxidizing agent. CO is created when an excess of C is burned in the presence of inadequate O2, with the O.N. of C being +2
C( excess )+O2→CO
If there is an excess of O2 in the combustion of C,CO2 is generated, with the O.N. of C being +4
C+O2( excess )→CO2
How do you count for the following observations?
(a) Though alkaline potassium permanganate and acidic potassium permanganate both are used as oxidants, yet in the manufacture of benzoic acid from toluene we use alcoholic potassium permanganate as an oxidant. Why? Write a balanced redox equation for the reaction.
(b) When concentrated sulphuric acid is added to an inorganic mixture containing chloride, we get colorless pungent smelling gas HCl, but if the mixture contains bromide then we get red vapour of bromine. Why?
(a) Alcoholic potassium permanganate is utilized as an oxidant in the production of benzoic acid from toluene for the following reasons.
(i) In a neutral medium, OH- ions are produced in the reaction itself. As a result, the cost of adding an acid or a base can be reduced.
(ii) Because both KMnO4 and alcohol are polar, they are homogenous. Because they a both organic molecules, toluene and alcohol are also homogenous. In a homogeneous medium, reactions can proceed more quickly than in a heterogeneous one. As a result, KMnO4 and toluene might react more quickly in alcohol.
For the reaction in a neutral medium, the balanced redox equation is as follows:
C6H5−CH3(l)+2MnO4−(alcoholic )→C6H5COO−(alcoholic)
+2MnO2(s)+H2O(l)+OH−(aq)
When concentrated H2SO4 is introduced to an inorganic bromide mixture, HBr is generated at first. With the formation of red bromine vapour, HBr, as a powerful reducing agent, lowers H2SO4 to SO2.
2NaBr+2H2SO4→2NaHSO4+2HBr2HBr+H2SO4→Br2+SO2+2H2O (red vapour)
When concentrated H2SO4 is added to an inorganic combination with chloride, a pungent-smelling gas (′HCl′ ) is produced. Because HCl is a poor reducing agent, it cannot convert H2SO4 to 2NaCl+2H2SO4→2NaHSO4+2HCl.
Identify the substance oxidized, reduced, oxidizing agent, and reducing agent for each of the following reactions:
(a) 2AgBr(s)+C6H6O2 (aq) →2Ag(s)+2HBr(aq)+C6H4O2(aq)
Ans:
Oxidized substance →C6H6O2
Reduced substance →AgBr
Oxidizing agent→AgBr
Reducing agent→C6H6O2
(b) HCHO(1)+2[Ag(NH3)2]+(aq)+3OH∘(aq)→2Ag(s)+HCOO−(aq)+4NH3(aq)+2H2O(I)
Ans: Oxidized substance → HCHO
Reduced substance→[Ag(NH3)2]+ Oxidising agent→[Ag(NH3)2]+ Reducing agent→HCH
(c) HCHO(I)+2Cu2+(aq)+5OH∘(aq)→Cu2O(s)+HCOO∘ (aq) +3H2O(I)
Ans: Oxidised substance→ HCHO
Reduced substance →Cu2+ Oxidising agent →Cu2+Reducing agent→ HGHO
(d) N2H4(I)+2H2O2 (I) →N2( g)+4H2O(I)
Ans: Oxidised substance→N2H4
Reduced substance→H2O2
Oxidising agent→H2O2
Reducing agent→N2H4
(e) Pb(s)+PbO2( s)+2H2SO4(aq)→2PbSO4( s)+2H2O(I)
Ans: Oxidised substance→ Reduced substance→PbO2
Oxidising agent→PbO
Justify giving reactions that among halogens, fluorine is the best oxidant and among hydrohalic compounds, hydroiodic acid is the best reductant.
F2 can also oxidize Cl to Cl2,Br−to Br2 and I−to I2
F2(aq)+2Cl(s)→2 F(aq)+Cl(g)
P2(aq)+2I(aq)→2 F(aq)+I2( s)
Cl2, Br2, and I2, on the other hand, are unable to convert F2. Halogens have an oxidizing power of I2<Br2<Cl2<F2. Fluorine, as a result, is the best halogen oxidant.
H2SO4 can be converted to SO2 using HI and HBr, but not with HCl or HF. HI and HBr are thus more effective reductants than HCl and HF
2HI+H2SO4→I2+SO2+2H2O
2HBr+H2SO4→Br2+SO2+2H2O
I can reduce Cu2+ to Cu+once more, whereas Br−cannot.
4I(aq)+2Cu2+(aq)→Cu2I2(f)+I2(aq)
As a result, among hydrohalic compounds, hydroiodic acid is the best reductant.
Hydrohalic acids' reducing power thus grows in the order of
HF<HCl<HBr<HI
Why does the following reaction occur?
XeO64−(aq)+2 F−(aq)→XeO3( g)+F2( g)+3H2O(l) What conclusion about the compound
NaXeO6 (of which XeO64 is a part) can be drawn from the reaction?
Because XeO64− oxidizes F−and F−decreases XeO64−, the stated reaction occurs.
XeO64−(aq)+2 F−(aq)→XeO3( g)+F2( g)+3H2O(l)
Xe 's oxidation number (O.N.) falls from +8 in XeO64 to +6 in XeO3, while F 's O.N. rises from
−1 in F−to O in F2
As a result, we can deduce that NaXeO64 is a more powerful oxidizer than F.
Consider the reactions:
(a) H3PO2(aq)+4AgNO3(aq)+2H2O(l)→H3PO4(aq)+4Ag(s)+4HNO3(aq)
(b) H3PO2(aq)+2CuSO4(aq)+2H2O(l)→H3PO4(aq)+2Cu(s)+H2SO4(aq)
(c) C6H5CHO(l)+2[Ag(NH3)2]+(aq)+3OH−(aq)→C6H5COO−(aq)+2Ag(s)+4NH3(aq)
(d) C6H5CHO(l)+2Cu2+(aq)+5OH−(aq)→ No change observed.
What inference do you draw about the behaviour of Ag+ and Cu2+ from these reactions?
In reactions (a) and (b),
Ag+ and Cu2+, respectively, act as oxidizing agents. Ag+ oxidizes C6H5CHO to C6H5COO−in reaction (c), but Cu2+ cannot oxidize C6H5CHO in reaction (d)
As a result, Ag+ is a more powerful oxidizing agent than Cu2+
Balance the following redox reactions by ion-electron method:
(a) MnO−(aq)+I−(aq)→MnO2( s)+I2( s)
Ans: Step 1 : The following are the two half reactions involved in the given reaction:
Half-reaction of oxidation I(aϕ)→I2( s)
Half-reaction of reduction MnO4 (aq) →MnO2(aq)
Step 2: We have the following equation for balancing ∣ in the oxidation half reaction:
2 F(aq)→I2( s)
To balance the charge, we add 2e−to the reaction's RHS.
2I(aq)−→I2( s)+2e−
Step 3: Mn 's oxidation state has decreased from+7 to+4 throughout the reduction half reaction.
MnO4−+3e−→MnO2(aq)
Step 4: Six O atoms are on the RHS and four O atoms are on the LHS in this equation. As a result, the LHS is given two water molecules.
MnO4(aq)−+2H2O+3e−→MnO2(aq)+4OH−
Step 5: By multiplying the oxidation half reaction by 3 and the reduction half reaction by 2 , we may equalize the quantity of electrons.
6I(aq)−→3I2( s)+6e−
2MnO4−(aq)+4H2O+6e−→2MnO2(aq)+8OH(aq)−
Step 6: When the two half reactions are added together, we get the net balanced redox reaction:
6I(aq)−+2MnO4(aq)+4H2O→2MnO2(aq)+8OH(aq)−
(b) MnO−(aq)+SO2(aq)→Mn2+(aq)+HSO4−(aq)
Ans: If we repeat the processes from part (a), we get the following oxidation half reaction:
SO2( g)+2H2O(l)→HSO4−(aq)+3H+(aq)
And the half-reduction reaction is as follows:
We get the net balanced redox reaction by multiplying the oxidation half reaction by 5 and the reduction half reaction by 2 , then adding them.
(c) H2O2(aq)+Fe2+(aq)→Fe3+(aq)+H2O(l)
Ans: Using the same techniques as in part (a), we get the following oxidation half reaction:
Fe2+(aq)→Fe3+(aq)+e−
And the half-reduction reaction is as follows:
H2O2(aq)+2H(aq)++2e−→2H2O(i)
We get the net balanced redox reaction by multiplying the oxidation half reaction by 2 and then adding it to the reduction half reaction:
H2O2(aq)+2Fe(aq)2++2H+(ac)→2Fe(2q)3++2H2O(i)
(d)Cr2O72−+SO2( g)→Cr3+(aq)+SO42−(aq)
Ans: Using the same techniques as in part (a), we get the following oxidation half reaction:
SO2( g)+2H2O(1)→SO4(2q)2+4H(aq)++2e−
And the half-reduction reaction is as follows:
Cr2O7(aq)2−+14H(aq)++6e−→Cr3+(aq)+7H2O(i)
We get the net balanced redox reaction by multiplying the oxidation half reaction by 3 and then adding it to the reduction half reaction:
Cr2O2⋅7(aq)+3SO2( g)+2H(aq)+→2Cr3+(a9)+3SO2−4(aq)+H2O(l)
What sorts of information can you draw from the following reaction?
(CN)2( g)+2OH(aa)−→CN(2aq)−+CNO(aq)−+H2O(i)
The carbon oxidation numbers in (CN)2,CN−,CNO−are +3,+2 and +4respectively.
These can be found as follows:
Let x be C 's oxidation number.
(CN)2
2(x−3)=0
∴x=3
CN∘
x−3=−1
∴x=2
CNO−
x−3−2=−1
∴x=4
The carbon oxidation number in various species is:
[CN]2( g)+2OH(aq)−→CN(aq )∗+CNO(aq)−+H2O(b)
In the preceding equation, the same chemical is being reduced and oxidized at the same time. Disproportionation reactions are those in which the same chemical is reduced and oxidized at the same time. As a result, the alkaline breakdown of cyanogen can be considered a disproportionation process.
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+,MnO2, and H+ ion. Write a balanced ionic equation for the reaction.
The following is a representation of the provided reaction:
Mn3+(aq)→Mn2+(aq)+MnO2( s)+H(aq)+
The half-equation for oxidation is:
Mn3++3(aq)→+MnO2( s)+4
By adding one electron to the oxidation number, the oxidation number is balanced as follows:
Mn3+(aq )→+MnO2( s)+e−
The charge is balanced by introducing 4H+ions in the following way:
Mn3+(aq)→+MnO2( s)+4H++e
2H2O molecules are added to balance the O atoms and H+ions as follows:
Mn(aq)3++2H2O→+MnO2(s)+4H++e−…..(i)
The hąlf-reduction equation is as follows:
Mn3+(aq)→Mn2+(aq)
By adding one electron to the oxidation number, the oxidation number is balanced:
Mn3+(aq)+e−→Mn2+(aq)
Combining equations I and (ii) yields the balanced chemical equation:
Mn3+(aq)+2H2O→+MnO2( s)+2Mn2+(aq)+4H+
Consider the elements: Cs,Ne,I and F
(a) Identify the element that exhibits only negative oxidation state.
Ans:
F has just a −1 negative oxidation state.
(b) Identify the element that exhibits only positive oxidation state.
Ans:
Cs has a positive oxidation state of +1
(c) Identify the element that exhibits both positive and negative oxidation states.
Ans: Both positive and negative oxidation states are present in my body. It has the following oxidation states:
−1,+1,+3,+5, and +7
(d) Identify the element which exhibits neither the negative nor does the positive oxidation state.
Ans: Ne has a zero-oxidation state. It doesn't have any oxidation states, either negative or positive.
Chlorine is used to purify drinking water. Excess of chlorine is harmful. The excess of chlorine is removed by treating with Sulphur dioxide. Present a balanced equation for this redox change taking place in water.
The following is a representation of the provided redox reaction:
Cl2( s)+SO2(aq)+H2O→Cl(aq)−+SO2−4(aq)
The half-reaction of oxidation is: +4
SO2(aq)→SO2−4(aq)
By adding two electrons to the oxidation number, the oxidation number is balanced:
SO2(aq)→SO2−4(aq)+2e−
The charge is balanced by introducing 4H+ions in the following way:
SO2(aq)→SO2−4(aq)+4H++2e−
2H2O molecules are added to balance the O atoms and H+ ions as follows: The charge is balanced by introducing 4H+ions in the following way:
SO2(aq)+2H2O(l)→SO2−4(aq)+4H++2e−
The half-reduction reaction is as follows:
Cl2( s)→Cl(aq)−
The chlorine atoms are balanced in the following way:
0−1Cl2( s)→Cl−(aq)
By adding electrons, the oxidation number is restored.
Cl2(s)+2e−→2Cl(aq)−…… (ii)
Combining equations I and (ii) yields the balanced chemical equation:
Cl2( s)+2SO2(aq)+2H2O(i)→2Cl(aq)−+SO2−4(aq)+4H+(aq)
Refer to the periodic table given in your book and now Ans: the following questions:
(a) Select the possible non-metals that can show a disproportionation reaction.
Ans: One of the reacting compounds must always contain an element that can exist in at least three oxidation states in disproportionation reactions.
(a) Because these elements can exist in three or more oxidation states, disproportionation reactions can occur.
(b) Select three metals that can show a disproportionation reaction.
Ans: Because these elements can exist in three or more oxidation states, disproportionation reactions can occur.
In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g. of ammonia and 20.00 g of oxygen?
For the above reaction, the balanced chemical equation is:
4NH3( g)+5O2( g)→4NO(g)+6H2O(g)
4×17 g5×32 g4×30 g6×18 g
=68 g=160 g=120 g=108 g
Therefore,
68 g of NH3 reacts with 160 g of O2
Thus, NH3 reacts with 160×1068 g of O2 23.53 g of O2
However, there is only 20 g of oxygen accessible.
As a result, O2 is the reaction limiting reagent (we used the amount of O2 to compute the weight of nitric oxide produced).
Hence, 160 g of O2 gives 120 g of NO 20 g of O2 gives 120×20160 g of N
Thus, 15 g of NO
As a result, you can get up to 15 g of nitric oxide.
Using the standard electrode potentials given in the Table 8.1, predict if the reaction between the following is feasible:
(a) Fe3(aq) and I−(aq)
Predict the products of electrolysis in each of the following:
(i) An aqueous solution of AgNO3 with silver electrodes
Ans: The reaction between Ag and Cu can be described as follows:
At the cathode, electrolysis can decrease either Ag+ions or H2O molecules. However,
Ag+ions have a larger reduction potential than H2O
Ag(aqi +e−→Ag(s);E0=+0.80 V
2H2O(l)+2e−→H2( g)+2OH(2q)−;E0=−0.83 V
As a result, at the cathode,
Ag+ions are decreased. At the anode, Ag metal or H2O molecules can also be oxidised. However, Ag molecules have a larger oxidation potential than H2O molecules.
Ag(s)→Ag(aq)++e−;E0=−0.80 V
2H2O(i)+2e−→H2( g)+2OH(aq)−;E0=−0.83 V
As a result, Ag metal oxidises at the anode.
(ii) An aqueous solution AgNO3 with platinum electrodes
Ans:
Pt is difficult to oxidise. As a result, at the anode, water is oxidised, releasing O2.
Ag+ions are reduced and deposited at the cathode. In aqueous solutions, H2SO4 ionises to give ___.
(iii) A dilute solution of H2SO4 with platinum electrodes
Ans: {H}^{+} and {SO}^{2}{ }_{4}$ ions.
H2SO4(aq)→2H(aq++SO4(aq)2−
At the cathode, electrolysis can decrease either H+ions or H2O molecules.
H+ions, on the other hand, have a greater reduction potential than H2O molecules.
2H(aq)++2e−→H2( g);E0=0.0 V
2H2O(aq)+2e−→H2( g)+2OH(aq)−;E0=0.83 V
As a result, H+ions are reduced at the cathode, releasing H2 gas.
The anode, on the other hand, can oxidise either two SO4 ions or two H2O molecules.
However, when SO2−4 is oxidised, more bonds are broken than when H2O molecules are oxidised.
As a result, the oxidation potential of SO2−4 ions is lower than that of H2O. As a result,
H2O is oxidised at the anode, releasing O2 molecules.
(iv)An aqueous solution of CuCl2 with platinum electrodes.
Ans:
CuCl2 ionises in aqueous solutions to produce Cu2+ and Cl−ions as Cu2+ and Cl−ions.
CuCl2(aq)→Cu2+(aq)+2Cl−
Cu2+ ions or H2O molecules can be reduced at the cathode during electrolysis.
Cu2+ on the other hand, has a greater reduction potential than H2O molecules.
Similarly, either Cl−or H2O is oxidised at the anode. The oxidation potential of
H2O is greater than the oxidation potential of 2Cl(aq) ∗→Cl2( g)+2e−;E0=−1.36 V
2H2O(1)→O2( g)+4H(2q)++4e−;E0=−1.23 V
However, due to over-voltage, oxidation of H2O molecules occurs at a lower electrode potential than that of Cl−ions (extra voltage required to liberate gas). As a result, at the anode, Cl−ions are oxidised, releasing Cl2 gas.
Arrange the following metals in the order in which they displace each other from the solution of their salts.
Al,Cu,Fe,Mg and Zh
A metal with a higher reducing power displaces a metal with a lower reducing power from its salt solution.
Al,Cu,Fe,Mg and Zn are the metals in order of increasing reducing power. As a result, we can conclude that Mg can evict Al from its salt solution, while Al cannot evict Mg. As a result, the following is the sequence in which the supplied metals displace each other from the solution of respective salts:
Mg>Al>Zn>Fe>Cu
Given the standard electrode potentials,
K+/K=−2.93 V,Ag+/Ag=0.80 V
Hg+/Hg−0.79
Mg2+/Mg=−2.37 V⋅
Cr3+/Cr=−0.74 V
Mg2+/Mg=−2.37 V⋅Cr3+/Cr=−0.74 V
Arrange these metals in their increasing order of reducing power.
The stronger the reducing agent is, the lower the electrode potential. As a result, the reducing power of the above metals is in ascending order:
Ag>Hg>Cr>Mg>K
Zn(s)+2Ag+(aq)→Zn2+(aq)+2Ag(s) takes place, further show
(i) which of the electrode is negatively charged,
(ii) the carriers of the current in the cell, and
(iii) individual reaction at each electrode.
Ans: The galvanic cell that corresponds to the given redox reaction looks like this:
Zn[Zn(aq)2+‖Ag(aq)+]Ag
(i) Because Zn2+ at this electrode, the remaining electrons concentrate on it, the Zn electrode is negatively charged.
(ii) Ions are the current carriers in cells.
(iii) The reaction at the Zn electrode can be represented as follows:
Zn(s)→Zn2+(aq)+2e−
The reaction at the Ag electrode can be represented as follows:
Ag+(aq)+e∘→Ag(s)
(iv) CuCl2 ionizes in aqueous solutions to produce Cu2+ and Cl− ions as:
CuCl2(aq)→Cu2+(aq)+2Cl−(aq)
Cu2+ ions or H2O molecules can be reduced at the cathode during electrolysis.
Cu2+(aq )+2e−→Cu(aq);E0=+0.34 V
H2O(i)+2e−→H2( g)+2OH−;E0=−0.83 V
Cu2+ ions are so reduced and deposited at the cathode.
The oxidation potential of H2O is greater than the oxidation potential of
2Cl(aq)−+Cl2(H)→2e−;E0=−1.36 V
2H2O(i)+O2( g)→4H+2( g)+4e−⋯;E0=−1.23 V
However, due to over-voltage, oxidation of H2O molecules occurs at a lower electrode potential than that of Cl−ions (extra voltage required to liberate gas).
Admissions Open for 2025-26

