Niels Bohr: The Molecule's Closest Companion
By Harshitha |
Date 01-10-2024
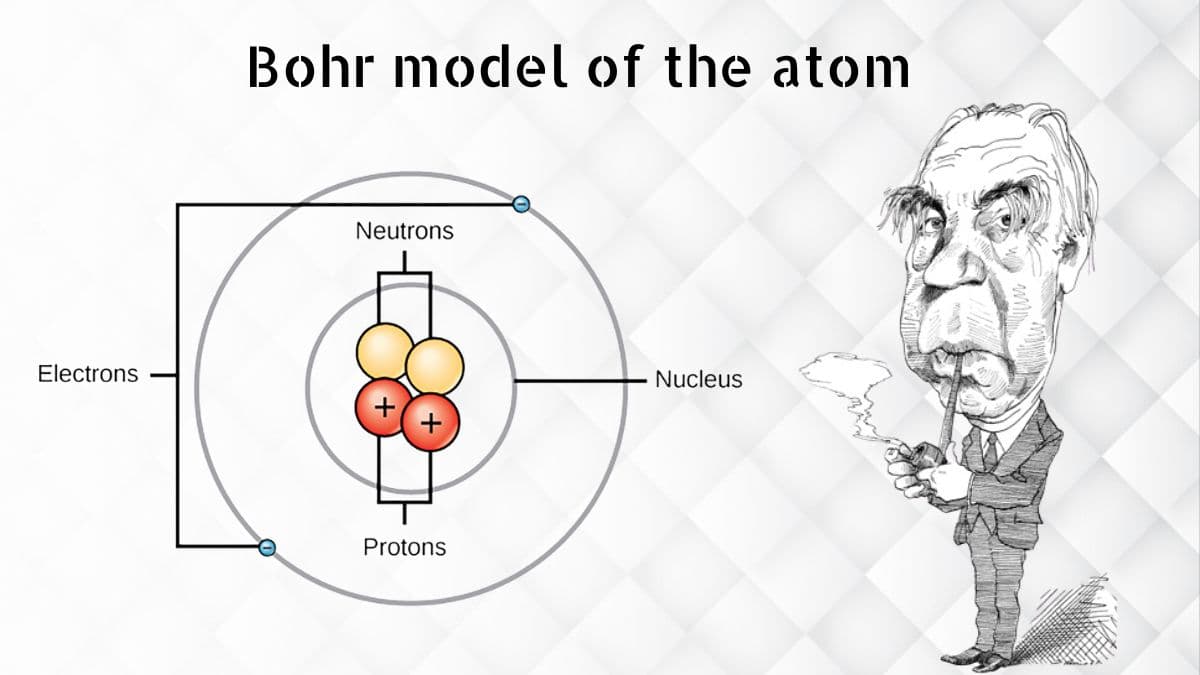
Table of Contents
- Who is Niels Bohr?
- Niels Bohr's Nuclear Model
- Niels Bohr's Nuclear Hypothesis
- Niels Bohr and the Intermittent Table
- Why Bohr's Work Matters
- Fun Realities About Niels Bohr
- Niels Bohr and Nuclear Design: A More profound Look
- Why Bohr's Model Was a Forward leap
- The Bohr Model and the Intermittent Table
- Bohr's Effect on Current Science
- Fun Realities About Niels Bohr
- Conclusion
- FAQs
Admissions Open for
Who is Niels Bohr?
Niels Bohr was a splendid researcher who carried on with quite a while in the past, in the mid twentieth hundred years. He was brought into the world in Denmark in 1885 and grew up to become quite possibly the main physicist ever. Bohr is most popular for his work on understanding the design of particles, the little structure blocks of everything around us.
However, what makes Bohr so extraordinary? Indeed, he concocted a groundbreaking thought regarding how molecules work, which fundamentally impacted the manner in which we see the world at the littlest scale. His hypotheses assisted researchers with understanding how iotas are assembled and the way that they act. How about we plunge into his amazing work and see what he found!
Niels Bohr's Nuclear Model
Before Bohr, researchers had a few thoughts regarding iotas, yet they didn't completely grasp them. Envision molecules as little, undetectable balls that make up everything around us. Bohr's model of the molecule resembles an exceptional outline that shows how these little balls are coordinated.
Bohr's nuclear model, presented in 1913, was a major leap forward. He recommended that molecules resemble a smaller than expected planetary group. In this model:
The Core: At the focal point of the particle is the core, which resembles the sun in our nearby planet group. The core is tiny however it contains the greater part of the molecule's mass. It is composed of protons and neutrons.
Electron Circles: Around the core are electrons, which are like planets circling the sun. These electrons move in unambiguous ways or "circles" around the core. Bohr said that electrons can exist in these circles and not in the middle between.
Bohr's model made sense of why particles emanate light when they are warmed. At the point when electrons assimilate energy, they leap to higher circles. At the point when they return to their unique circles, they discharge energy as light. Therefore we see various shades of light from sparkling molecules!
Niels Bohr's Nuclear Hypothesis
Niels Bohr's nuclear hypothesis is a piece like a bunch of rules for how molecules work. Here are a few significant pieces of his hypothesis:
Quantized Circles: As per Bohr, electrons can involve specific permitted circles around the core. These circles have explicit energy levels. Electrons can move between these circles by retaining or transmitting energy.
Energy Levels: Each circle or energy level resembles a stepping stool. Electrons can remain on the rungs of this stepping stool. The farther an electron is from the core, the higher the energy level.
Soundness of Electrons: Electrons in these particular circles are steady and don't lose energy. Just when they bounce between circles do they retain or produce energy.
Bohr's hypothesis was vital on the grounds that it made sense of how particles produce light and how they interface with different molecules. It likewise assisted researchers with understanding the shades of light in firecrackers and neon signs.
Niels Bohr and the Intermittent Table
The occasional table is a diagram that coordinates every one of the known components (the kinds of particles) that shows their properties. Before Bohr's work, researchers realized a ton about components however didn't comprehend their construction profoundly.
Bohr's nuclear model assisted researchers with understanding the reason why components have various properties and how they respond with one another. By utilizing Bohr's model, researchers could more readily comprehend the plan of electrons in molecules, which is pivotal for knowing how components bond and communicate.
For instance, components in a similar section of the occasional table frequently have comparative properties. Bohr's model made sense of this by showing what the quantity of electrons and their plan mean for a component's way of behaving.
Why Bohr's Work Matters
Niels Bohr's work was historic on the grounds that it changed how we might interpret the molecule. Here's the reason his disclosures are so significant:
Better Nuclear Models: Bohr's nuclear model was an improvement over past models. It made sense of numerous things that researchers couldn't comprehend previously.
Establishment for Future Science: Bohr's thoughts laid the preparation for future revelations in physical science and science. His work prompted the improvement of quantum mechanics, a part of science that makes sense of the way of behaving of tiny particles.
Seeing Light: Bohr's model made sense of how iotas transmit light, which is significant for some advancements, including lasers and Drove lights.
Synthetic Responses: By understanding how electrons are organized, researchers can anticipate how various components will respond with one another, which is pivotal for making new materials and meds.
Fun Realities About Niels Bohr
Family Custom: Niels Bohr's dad, Christian Bohr, was likewise a researcher. Nielss emulated his dad's example and became quite possibly the best physicist.
Nobel Prize: Bohr won the Nobel Prize in Physical science in 1922 for his work on nuclear hypothesis. This is quite possibly the greatest honor a researcher can get!
Bohr's Foundation: Bohr established the Organization for Hypothetical Physical Science in Copenhagen, which turned into a significant place for research in quantum mechanics.
Niels Bohr and Nuclear Design: A More profound Look
Niels Bohr's commitments to nuclear construction address a critical jump in how we might interpret iotas. Before Bohr, researchers had a fundamental thought of molecules yet missed the mark on point by point image of their inner design. Bohr's work in the mid twentieth century changed how we see iotas and established the groundwork for the present day nuclear hypothesis.
The Core: At the focal point of a molecule is the core, a thick center that contains protons and neutrons. Protons are emphatically charged particles, while neutrons have no charge. The core is minuscule contrasted with the whole particle, yet it contains virtually the iota's all's mass.
Electron Circles: Encompassing the core are electrons, which are a lot more modest than protons and neutrons. Bohr suggested that electrons move in fixed ways or circles around the core, similar to how planets circle the sun. These circles are at various energy levels, which Bohr alluded to as "quantized" energy levels.
Energy Levels: Each circle or energy level relates to a particular measure of energy. Electrons can exist in these particular circles and can't be found between them. This thought was a significant takeoff from prior models, which proposed that electrons could exist anyplace around the core.
Quantized Energy: As indicated by Bohr's model, electrons can move between these circles by engrossing or producing energy. At the point when an electron ingests energy, it leaps to a higher circle. At the point when it discharges energy, it falls back to a lower circle and radiates light. For this reason iotas produce light of explicit varieties when they are warmed or invigorated.
Why Bohr's Model Was a Forward leap
Bohr's nuclear model tackled a few issues that past models couldn't make sense of. Here's the reason his model was so weighty:
Making sense of Nuclear Spectra: Before Bohr, researchers saw that iotas discharged light in unambiguous examples, called nuclear spectra. These examples were challenging to make sense of with more established models. Bohr's model effectively made sense of these examples by showing that electrons possess explicit circles and can retain or emanate specific measures of energy.
Soundness of Particles: Bohr's model likewise made sense of why electrons don't wind into the core. As indicated by traditional physical science, an electron ought to persistently lose energy and ultimately fall into the core. Bohr's quantized circles showed that electrons are steady in their circles and possibly lose or acquire energy when they hop between levels.
Figuring out Compound Responses: Bohr's model furnished knowledge into how iotas bond with one another. By understanding the course of action of electrons in various circles, researchers could more readily anticipate how molecules would associate. This was vital for making sense of compound responses and holding.
The Bohr Model and the Intermittent Table
The intermittent table coordinates components in light of their properties and nuclear design. Bohr's model essentially influenced how researchers figure out the intermittent table:
Electron Setup: Bohr's model made sense of the electron arrangement of components. The plan of electrons in various circles or energy levels decides a component's synthetic properties. For instance, components in a similar segment of the occasional table have comparative properties since they have comparative electron setups.
Occasional Patterns: By understanding how electrons are organized, researchers could all the more likely anticipate patterns in the intermittent table, like nuclear size and reactivity. For example, components in a similar line of the occasional table have electrons filling a similar energy level, while those in a similar section have comparative external electron setups.
Compound Bonds: Bohr's model likewise given a premise to understanding how iotas cling to shape particles. The plan of electrons decides how particles will bond, which is fundamental for figuring out synthetic responses and the arrangement of mixtures.
Bohr's Effect on Current Science
Niels Bohr's nuclear model was not the last word on nuclear design but rather it was a vital step in the right direction. It was subsequently refined by different researchers, prompting the improvement of quantum mechanics — a further developed hypothesis that portrays the way of behaving of electrons in particles.
Bohr's thoughts regarding quantized energy levels laid the foundation for this new area of science. Quantum mechanics expands on Bohr's model by presenting more refined ideas, like the wave idea of electrons and the vulnerability guideline.
Notwithstanding these headways, Bohr's model remains a basic piece of nuclear hypothesis. It gives a reasonable and basic method for grasping the design of particles and is as yet educated to understudies as a prologue to nuclear construction.
Fun Realities About Niels Bohr
-
Nobel Prize: Niels Bohr won the Nobel Prize in Physics in 1922 for his work on atomic structure. This prestigious award recognized his groundbreaking contributions to our understanding of the atom.
-
Bohr’s Institute: Bohr founded the Institute for Theoretical Physics at the University of Copenhagen. This institute became a leading center for research in quantum mechanics and attracted many prominent scientists.
-
Legacy: Bohr’s work not only advanced our understanding of atomic structure but also inspired future generations of scientists. His ideas continue to influence research in physics and chemistry.
Conclusion
Niels Bohr atomic model was a major milestone in the study of atoms. His ideas about electron orbits, quantized energy levels, and atomic spectra revolutionized our understanding of Niels Bohr atomic theory. By explaining how atoms emit light and interact with each other, Bohr’s model provided valuable insights into the nature of matter.Niels Bohr atomic theory and quantum mechanics laid the foundation for modern physics. His work remains a crucial part of scientific education and continues to influence research in physics and chemistry. Niels Bohr’s legacy as a pioneering scientist is firmly established, and his model of the atom still holds an important place in our understanding of the microscopic world.
FAQs
What is Niels Bohr famous for?
He adapted Rutherford's nuclear structure to Max Planck's quantum theory and created the Bohr model, the most widely accepted model of the atom. In 1922, Bohr was awarded the Nobel Prize in Physics for his research and contributions on the structure of an atom.
What did Bohr's theory do?
Bohr proposed that electrons do not radiate energy as they orbit the nucleus, but exist in states of constant energy that he called stationary states. This means that the electrons orbit at fixed distances from the nucleus (see below). Bohr's work was primarily based on the emission spectra of hydrogen.
Who discovered the Bohr atom?
In July of 1913, Danish physicist Niels Bohr published the first of a series of three papers introducing this model of the atom, which became known simply as the Bohr atom.
Did Bohr agree with Einstein?
Despite their differences of opinion and the succeeding discoveries that helped solidify quantum mechanics, Bohr and Einstein maintained a mutual admiration that was to last the rest of their lives. Although Bohr and Einstein disagreed, they were great friends all their lives and enjoyed using each other as a foil.
Share info about niels-bohr with everyone and don’t forget to leave a comment below
Related Blogs
Atomic Structure: Get to know about Atomic Structure by Niels Bohr along with interesting facts through our new article!
CBSE Schools In Popular Cities
- CBSE Schools in Bangalore
- CBSE Schools in Mumbai
- CBSE Schools in Pune
- CBSE Schools in Hyderabad
- CBSE Schools in Chennai
- CBSE Schools in Gurgaon
- CBSE Schools in Kolkata
- CBSE Schools in Indore
- CBSE Schools in Sonipat
- CBSE Schools in Delhi
- CBSE Schools in Rohtak
- CBSE Schools in Bhopal
- CBSE Schools in Aurangabad
- CBSE Schools in Jabalpur
- CBSE Schools in Jaipur
- CBSE Schools in Jodhpur
- CBSE Schools in Nagpur
- CBSE Schools in Ahmednagar
- CBSE School In Tumkur

Call Us to know more about Orchids
Swipe Up

















