Atom: The Building Block of Everything
By Harshitha |
Date 05-10-2024
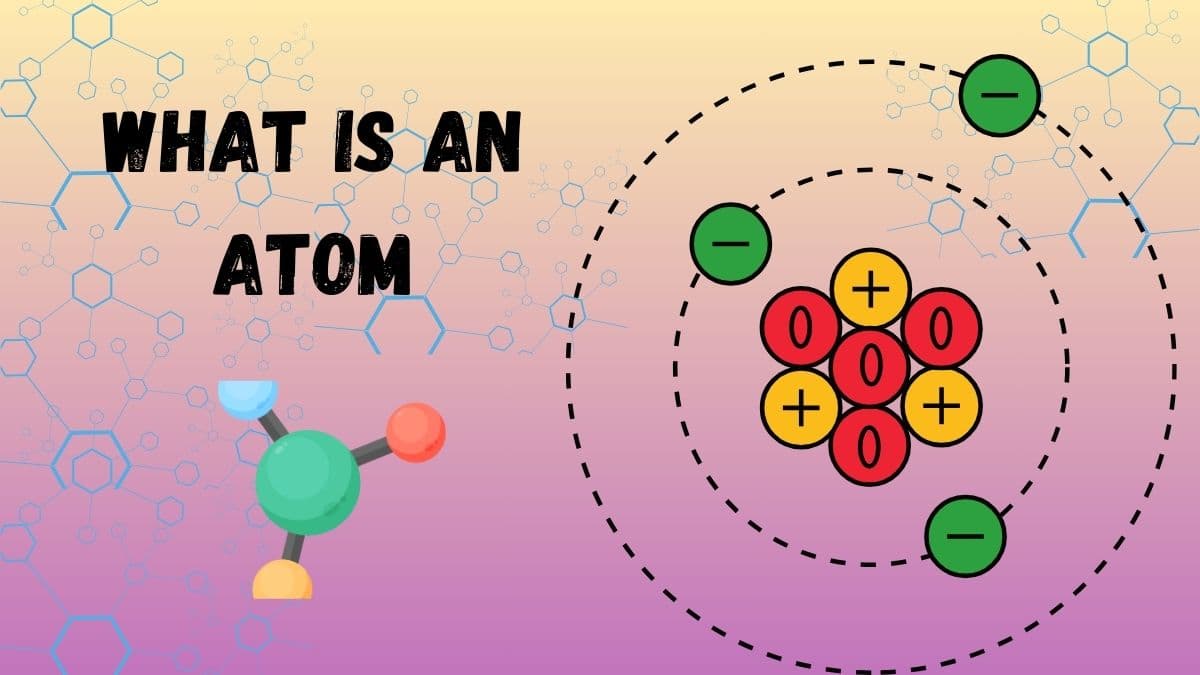
Table of Contents
Admissions Open for
What is an atom?
An atom is the small building block of matter with properties still retaining those of an element. That means, it is so minute, you cannot see it with your eyes, yet everything one sees, touches, or even smells is still made up of atoms. Everything in the universe constitutes these atoms, including you and me. The atoms are really small; if lined up side by side, a million atoms would be as wide as a strand of hair. As minute as they might be, atoms are very important because they make up all the elements on the periodic table.
Atoms are much like miniature solar systems. The middle of the atom, the nucleus, contains protons and neutrons. Orbiting around the nucleus are electrons; these zoom around in orbits much like the ways that planets orbit the sun. We call that the atomic structure of an atom. Because we cannot perceive atoms with our eyes, scientists have special tools that allow them to study atoms and learn about their amazing properties.
What is Atomic Structure?
Structure of an atom- the term atomic structure denotes how the parts of an atom are put together. Like in every different house, the room and the walls are different, similarly different atoms are made up by their different parts. To understand, we would break it down to see what makes up an atom:
Nucleus: This is the center of the atom; it would be the sun in terms of our solar system. It is compact and is composed of two types of particles: protons, and neutrons. The nucleus is highly small in relation to the atom but it contains nearly all the mass of the atom.
Protons: These are positively charged atomic nuclei, which each atomic number possesses. An atom is what it is due to the number of protons which are contained in it. If an atom contains one proton, it will be hydrogen; if it has six protons, it will be carbon. The number of protons is essential in the identification of the atom. The more the protons in an atom, the heavier and the more complex the atom is.
Neutrons: Neutrons are neutral, and carry no charge. They reside with the protons in the nucleus and contribute to the stability of the nucleus. Neutrons are like the adhesive that holds the nucleus together. Without neutrons, the protons would strongly repel each other and the nucleus would break apart.
Electrons: Elec-trons are negatively charged particles that whizz around the nucleus of an atom in areas called electron clouds or shells. Even though they have a negative charge, they don't crash into the nucleus because they are just moving so fast! Electrons are really, really smaller than protons and neutrons, and they race around the nucleus at amazing speeds. The arrangement of electrons within an atom's electron clouds or shells determines the atom's chemical properties. That would imply atoms have the greater effect of creating between themselves interactions.
The constituent protons, neutrons, and electrons, therefore, impart an atom with a unique atomic structure. One can say in every atom, there is another mini universe inside it: the nucleus is in the center and electrons dance around that center. The Atomic structure of any element imparts the differences characteristic of one from another and gives them distinct properties.
What is Atomic Number?
Atomic number is an atom's identity card. It tells us about the number of protons in the nucleus of an atom. Remember how we said the number of protons decides which element the atom is? That's where the atomic number comes in. The atomic number is unique to each element, and it is what makes one element different from another.
That is to say, for example, the atomic number of hydrogen is one because it has one proton, while that of carbon is six because it contains six protons. All things in the periodic table have an atomic number. The Periodic Table is arranged according to an atomic number; therefore, you can easily get how many protons an element has if you look at its position on the table.
Knowing the atomic number of an element helps the scientist further understand the kind of atom they are dealing with and how that atom will behave in different situations. An example could be that elements that have similar atomic numbers often have similar properties; this is why the elements are grouped in the periodic table. The atomic number is amongst the most fundamental information about an atom since it can tell us what element it is and how it may react with another element.
What is an Atomic Mass Unit?
Now, according to that, let me explain to you what an atomic mass unit is, which is usually abbreviated as AMU. An atomic mass unit is used for measuring the minute substances of atoms. The reason behind this is that the atoms are so tiny, and their mass is also very tiny; it should be measured, so scientists derived a special unit which is the atomic mass unit.
One atomic mass unit is defined as one-twelfth the mass of a carbon-12 atom. Now, this probably sounds very confusing, but it's simply just giving us something with which to measure atoms. The reason the carbon-12 atom has been selected to be the standard is because it is an extremely stable and abundant isotope, which makes it a very good reference point to measure atomic masses.
For example, a proton has approximately 1 atomic mass unit mass. A neutron also has the same mass as that of a proton. Electrons are so light that their masses are almost negligible in comparison to protons and neutrons. It thus means that when talking about mass, an atom is often considered in the number of protons and neutrons making up its nucleus.
Normally, what we refer to as the mass of the atom is just the sum of the masses of all protons and neutrons present within the nucleus, in atomic mass units, so as to obtain the atomic mass of that element. For example, the atomic mass of carbon is approximately 12 atomic mass units because it has six protons and six neutrons. The atomic mass gives an important insight into just how heavy one atom is compared to another atom.
Why is the Atomic Mass Unit Important?
The atomic mass unit is important in that it has enabled scientists to compare the masses of different atoms with much ease. Without the atomic mass unit, quite a number of elements in relation to each other would not have been understood. In this respect, the atomic mass unit provided a standard way of measuring and comparing masses of atoms; thus, it made studying the properties of various elements simple.
For instance, considering oxygen has an atomic mass of about 16 atomic mass units, while hydrogen has an atomic mass of about 1 atomic mass unit, we realize an oxygen atom is much heavier compared to a hydrogen atom. This helps in chemistry when scientists have to mix these or other elements together. Using the atomic mass unit helps them to tell how much of each element they need.
The atomic mass unit serves to enable the scientists to have an idea about the relative abundance of the different elements in the universe. For instance, hydrogen is considered to be the most abundant element due to its lightest and simplest mass approaching nearly 1 atomic mass unit. On the other hand, the heavier elements are found less abundant because of higher atomic masses due to their complexity and further energy requirement for their formation.
How do atoms form molecules?
Atoms are seldom seen just alone; they love to bond with other atoms to form molecules. A molecule is merely a group of atoms stuck together: for example, water consists of two hydrogen atoms and one oxygen atom. When these atoms are put together, they form a molecule of water. They can be as simple as a few atoms, like water, or as complicated as thousands of atoms, as in proteins and DNA.
The atomic constitution of atoms, along with the number of electrons inside them, dictates the way in which they bond with other atoms. The atoms may start sharing, giving away, or even taking electrons from other atoms to form a molecule. This forms an interesting world around us because all this is due to this process. For instance, the way in which atoms are bonded together describes whether something is a solid, liquid, or gas. It also describes the reactivity versus stability of a substance.
The atoms can form several kinds of bonds: covalent bonds, ionic bonds, and metallic bonds. Covalent bonds are formed when atoms share electrons, ionic when one atom loses an electron to another atom, and metallic when atoms share electrons in a "sea" of electrons. Each of these bonds has its own characteristics, the type of bond between atoms is determined by the atomic structure of the atoms concerned and by the way their electrons are arranged.
What is the Periodic Table?
It is that enormous chart in which elements appear to be arranged according to increasing atomic numbers. All of those little squares on the periodic table perhaps contain the same information on an element, such as the atomic number, the symbol, and sometimes atomic mass in atomic mass units. The periodic table forms one of the most important principles of chemistry due to the fact that it assists in understanding a lot about the properties of elements and how they relate to certain properties.
The periodic table is designed by the use of arrangement in rows, periods, and columns, groups. Elements falling in the same group exhibit similar chemical properties on account of their having the same number of electrons in their outer shell. For example, elements in group 1, such as hydrogen and lithium, are highly reactive because each contains one electron in its outer shell.
The periodic table is color-coded so as to reflect various kinds of elements that include metals, nonmetals, and metalloids. Metals are usually shiny and good conductors of electricity, whereas on the contrary, nonmetals are generally dull and poor conductors. Metalloids have properties of both metals and nonmetals. The periodic table is an essential tool for scientists since it helps them predict the way elements will behave in different situations.
Why are Atoms Important?
Well, atoms are everything in the universe! Without atoms, there would be no stars, no planets, no trees, and no you! Atoms are the building blocks of all matter, and learning about them helps scientists understand how the world works.
But atoms are also important in that they enable scientists to devise new materials and technologies. The understanding of the atomic structure of various elements helps scientists create new materials with peculiar properties, such as stronger metals or more efficient batteries. Atoms are also invaluable in medicine, where scientists apply their knowledge of atoms in devising new medicines and treatments against diseases.
Besides, atoms play a great role in energy production. In a nuclear power plant, energy produced during the process of atomic fission is utilized to generate electricity. Such a process, which is called nuclear fission, releases a huge amount of energy because it involves breaking the strong bonds holding the nucleus of an atom together. It has also contributed to the discovery of nuclear fusion-the process which gives the energy of the Sun and now promises a virtually unlimited supply of clean energy.
Conclusion
Atoms are a fabulous world. Everything we see, touch, and experience are made by these atoms. From the food we eat to the air we breathe, atoms are everywhere. An understanding of atoms by knowing atomic mass units, atomic numbers, and atomic structure is important. Though atoms are small, they have a big impact on our lives-from mixing up a batch of cookies to building a rocket ship-you're working with atoms. And the next time you see a tree, a car, or even your hand, think of the trillions and trillions of tiny atoms working in concert to build up this wonderful world that we find ourselves in.
FAQs
1. What is Nuclear fission?
Nuclear fission is a process in which a heavy atomic nucleus is divided into smaller nuclei with the emission of energy. It is applied to the production of electricity in nuclear power stations.
2. Can atoms be destroyed?
Chemical reactions allow atoms to be rearranged but not destroyed. Nuclear reactions, on the other hand, are a different class altogether; atoms may be converted into another element.
Like what you read ? Do share this article with someone who needs to know everything about atoms.
Related Blogs
Father of Atom: Get to know about The Father of Atom, John Dalton through our latest article!
Proton: Discover interesting information about Proton; the building block of an atom through our latest article!
CBSE Schools In Popular Cities
- CBSE Schools in Bangalore
- CBSE Schools in Mumbai
- CBSE Schools in Pune
- CBSE Schools in Hyderabad
- CBSE Schools in Chennai
- CBSE Schools in Gurgaon
- CBSE Schools in Kolkata
- CBSE Schools in Indore
- CBSE Schools in Sonipat
- CBSE Schools in Delhi
- CBSE Schools in Rohtak
- CBSE Schools in Bhopal
- CBSE Schools in Aurangabad
- CBSE Schools in Jabalpur
- CBSE Schools in Jaipur
- CBSE Schools in Jodhpur
- CBSE Schools in Nagpur
- CBSE Schools in Ahmednagar
- CBSE School In Tumkur

Call Us to know more about Orchids
Swipe Up


.jpg&w=1920&q=80)













